
We test annually for the following parameters: pH, dissolved oxygen, conductivity, turbidity, temperature, total Kjeldahl nitrogen, alkalinity, ammonium, nitrate, nitrite, total phosphorus, orthophosphate, Escherichia coli (E. coli), and Enterococci.
Staff and volunteers utilize meters purchased from the HACH Company out of Loveland, Colorado, to conduct testing. The HACH HQ40D meter is used to test dissolved oxygen, conductivity, and temperature. The HACH 2100Q turbidimeter tests turbidity. The IntelliCAL(TM) pH, model PHC201, is used to test pH levels. Thanks to a grant from the Maine Outdoor Heritage Fund, which allowed the Commission to purchase advanced bacteria monitoring equipment from IDEXX Laboratories, the processing of E. coli and Enterococci samples moved in-house in 2022. This move has allowed the SRCC to expand our bacteria monitoring efforts to new sites and to obtain results faster, allowing for a better response time when notifying municipalities of concerning bacteria levels at popular recreation sites. In the 2023 season, the SRCC will launch a pioneering effort using environmental DNA (eDNA) isolated from water samples with high bacteria levels to determine the probable source of contamination. Samples collected to test for alkalinity, ammonium, nitrate, nitrite, total phosphorus, and orthophosphates are frozen at the SRCC office and transported monthly to the Water Resources Research Center at the University of New Hampshire for processing.
The following explanations describe each of these parameters and how the valuable information they provide is important both individually, and in combination with the others.
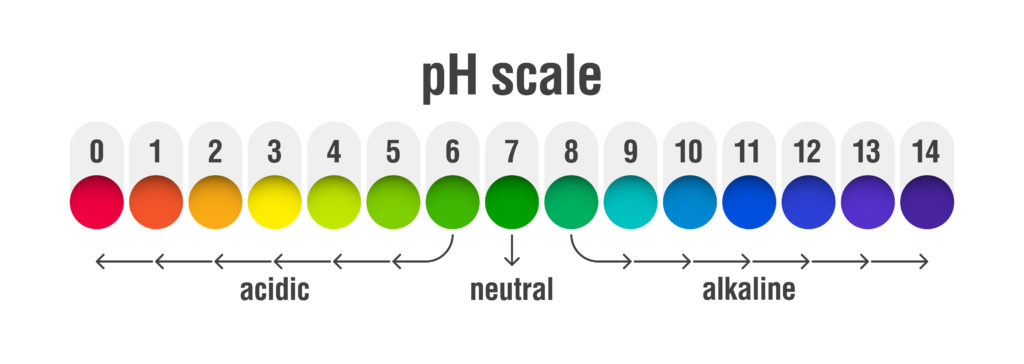
pH value scale chart for acid and alkaline solutions, acid-base balance infographic
The term pH means “potential of hydrogen” and is measured on a 1.0 to 14.0 scale in order to determine the acidity or alkalinity of a substance. Specifically, this scale is measuring the concentration of hydrogen ions (H+) and hydroxyl ions (OH-) which are both contained in water. (H+ + OH- = H20) Sometimes these ions are floating free and other times they are bound together with other ions such as sodium (Na+) or chloride (Cl-). Whenever you have more hydrogen ions floating free all by themselves, in comparison with hydroxyl ions, the water would be considered acidic and would have a pH of less than 7.0. At a pH of exactly 7.0 (neutral) the concentration of both free hydrogen ions and hydroxyl ions is equal. Whenever you have more hydroxyl ions floating free, compared with hydrogen ions, the water would be considered alkaline and would have a pH of more than 7.0.
Of course, all of this discussion so far is happening right before our eyes and yet we can’t see a thing. All we know is that if the water becomes too acidic or too alkaline, the fish and wildlife will begin to suffer. What is important to understand is that everything that our rivers come in contact with affects the pH scale. These waters flow along and through agricultural fields, lawns, roadways, and woodlands to name a few. All of these areas have their own ions we can’t see that have a natural tendency to bind with either the free hydrogen or hydroxyl ions in our rivers. Generally speaking, the ability of aquatic organisms to complete a life cycle greatly diminishes as pH falls below 5.0 or exceeds 9.0. The ideal range is between 6.5 and 8.2.
Key Takeaways:
Non-bonded oxygen molecules in water https://www.fondriest.com/environmental-measurements/parameters/water-quality/dissolved-oxygen
There are many living organisms in our rivers that require oxygen to survive. That oxygen is available to them in a gaseous state and is called dissolved oxygen. This amount of oxygen is commonly expressed as a concentration in terms of milligrams per liter (mg/L), or as a percent saturation (% sat). Milligrams per liter is the amount of oxygen in a liter of water. Percent saturation is the amount of oxygen in a liter of water relative to the total amount of oxygen that the water can hold at that temperature. There are many ways that oxygen can find its way into the water. The primary methods are through contact with the atmosphere and photosynthesis.
Accurate dissolved oxygen readings are dependent of temperature, atmospheric pressure, and salinity. Cold water has the ability to hold more oxygen versus warmer water. Increased atmospheric pressure also increases waters ability to hold more oxygen. Salinity, on the other hand, decreases the waters solubility. The amount of dissolved oxygen in the water is in direct relation to that waters ability to support aquatic organisms. Water with very low dissolved oxygen content (less than 5 mg/L), is usually caused by too much or improperly treated organic wastes, and does not support fish or similar organisms. Dissolved oxygen is essential for basic metabolic processes of most plants and animals. Oxygen is also consumed by bacteria decomposing dead plants and animals.
Dissolved oxygen levels rise from morning through the afternoon as a result of photosynthesis, reaching a peak in late afternoon. Photosynthesis stops at night, but plants and animals continue to respire and take in oxygen. As a result, dissolved oxygen levels fall to a low point just before dawn.
Depletions in dissolved oxygen can cause major shifts in the kinds of aquatic organisms found in water bodies. Species that can not tolerate low levels of dissolved oxygen — mayfly nymphs, stonefly nymphs, caddisfly larvae, beetle larvae — will be replaced by a few kinds of pollution-tolerant organisms, such as worms and fly larvae. Nuisance algae and anaerobic organisms (those that can live without oxygen) may also become abundant in waters with low levels of dissolved oxygen.
Key Takeaways:
https://www.fondriest.com/environmental-measurements/parameters
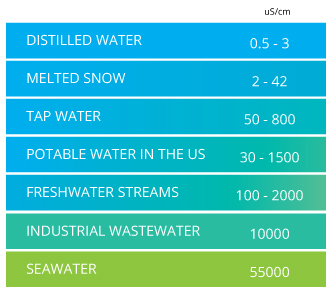
Conductivity is a measure of the ability of water to pass an electrical current. Because dissolved salts and other inorganic chemicals conduct electrical current, conductivity increases as salinity increases. Organic compounds like oil do not conduct electrical current very well and therefore have a low conductivity when in water. Conductivity is also affected by temperature: the warmer the water, the higher the conductivity.
Conductivity is useful as a general measure of water quality. Each water body tends to have a relatively constant range of conductivity that, once established, can be used as a baseline for comparison with regular conductivity measurements. Significant changes in conductivity could then be an indicator that a discharge or some other source of pollution has entered the aquatic resource.
Significant changes (usually increases) in conductivity may indicate that a discharge or some other source of disturbance has decreased the relative condition or health of the water body and its associated biota. Generally, human disturbance tends to increase the amount of dissolved solids entering waters which results in increased conductivity. Water bodies with elevated conductivity may have other impaired or altered indicators as well. As seasons change, conductivity will change as well due to water flow fluctuations: The lower the water level, the higher the concentration of salt.
Key Takeaways:

If you are trying to determine how turbid a substance is, you are measuring the clarity in a fluid. The greater the turbidity, the murkier the water. Higher levels of turbidity are usually caused by turbulent flow picking up large quantities of particulates, such as after a storm event or areas of erosion, both natural and man made. Other causes are waste discharge, urban runoff, abundant bottom feeders that stir up bottom sediments, or algal growth.
These high levels of suspended particles, which absorb heat from the sun, increase the water’s temperature and thus cause oxygen levels to fall. The lower oxygen levels have an effect on photosynthesis which in turn has an additional effect on lower oxygen levels. Suspended solids can clog fish gills, reduce growth rates, decrease resistance to disease, and prevent egg and larval development of aquatic life. Particles can also gather at the bottom of waterways and smother the eggs of fish and aquatic insects.
Key Takeaways:
Temperature is determined by the average kinetic energy (energy obtained by being in motion) in the molecules of the substance being measured. In other words, the faster the molecules are moving the more energy they have and the higher temperature. Slower molecules have less energy and therefore a lower temperature.
The metabolic rates of organisms increase with increasing water temperature. An increased metabolism increases the need for oxygen. Temperature also influences the amount of oxygen dissolved in water and the rate of photosynthesis by algae and larger aquatic plants.
Activities that can affect temperature include industrial discharge of water used to cool machinery (also known as thermal pollution), the cutting of trees that once shaded the water, and soil erosion which increases the suspended solids making the water more turbid.
When the water temperature increases, so too does the rate of photosynthesis and plant growth. The more plants that grow means that more plants will die. As the plants die, they are decomposed by bacteria that consumes oxygen.
Organisms that thrive in cooler water temperature (13° C and below) can include trout and mayfly nymphs. Those that prefer warmer waters (20° C and above) are bass and numerous plant life. The middle range (13° C to 20° C) supports salmon, trout, water beetles, and limited plant life. There are few organisms that can tolerate extreme heat or cold.
Key Takeaways:
Nitrogen
All plant and animal tissues require nitrogen for growth. Approximately 80% of the volume of the Earth’s atmosphere is made up of nitrogen. There is a constant nitrogen cycle that occurs on Earth between the air and the soils. Throughout that cycle, nitrogen takes on many forms.
Nitrogen can exist at nitrate (NO3), and as a nitrite (NO2). Nitrates are an important part of fertilizers used in agriculture and they easily leach from soils. Nitrites, unlike nitrates, are toxic to plants in great concentrations. Bacteria found naturally in soils use nitrites in the conversion process of Ammonia Nitrogen (NH4) into beneficial nitrates.
Total Kjeldahl nitrogen (TKN) is a combined measurement of all three forms of nitrogen discussed (NO3, NO2, and NH4).
Phosphorus
The element phosphorus exists in many different forms. In one form it is poisonous and can burn our skin on contact. In another, it exists as part of our DNA structure. Phosphorus can also be found within our water supply in the form of phosphate (PO-4-P).
Phosphorus is considered an essential element of life. Phosphorus provides plants and animals with necessary nutrients in order to undergo metabolic processes. Of course, there can be too much phosphorus within a water supply which can lead to an “algal bloom.” An algal bloom is an indicator of cultural eutrophication. Cultural eutrophication is the “over-enrichment of aquatic ecosystems caused by human activity, such as industrial pollution, septic tank leachate, or agriculture.” When waters become over enriched there is an increase in the levels of nutrients such as phosphates (or nitrates) which lead to a rapid increase in plant growth. Our waterways undergo a normal aging process in which plant matter naturally dies and is decomposed by organisms within the water. Whenever you have too much plant growth, the decomposing organisms (which need oxygen to survive) essentially are working in overdrive and begin to use up available oxygen levels. Low oxygen levels lead to death of organisms including fish. This aging process occurs constantly but at a slow rate. Cultural Eutrophication provides an explanation of how human influences can adversely affect a natural system.
Key Takeaways: Total Kjeldahl Nitrogen (TKN) and Total Phosphorus (TP)
E. coli
Group of bacteria such as Escherichia coli, Helicobacter pylori or salmonella 3D rendering illustration. Microbiology, medical, bacteriology, biology, science, medicine, infection concepts.
Whether we know it or not, our intestines are where a tiny bacterium called Escherichia coli (a type of fecal coliform) calls home. Actually, E. coli lives in the intestines of all warm blooded mammals. Our program tests for this bacteria as a possible indicator that human waste is entering the water supply. There are many ways that this can happen, including swimmers at a beach, campers who don’t properly bury their waste, or a failing septic system. E. coli itself is not usually pathogenic (disease-producing), there are, however, some strains that can cause gastrointestinal disturbances. Whenever one is exposed to excessive amounts of E. coli colonies through water contact (bacteria can enter the body through cuts on the skin or through the nose, mouth, or ears), there can be a variety of ways humans can react, including fever, vomiting, or ear infections.
When you have high fecal coliform counts (beginning at 200 colonies per 100 mL of water), a person swimming is at a greater risk of developing an adverse reaction to the present bacteria.
In order to obtain an accurate assessment of the bacteria count, five samples are taken at one time for testing. Current State of Maine standards require that E. coli results from five samples must not exceed 126 colonies per 100 milliliters of water in order to be considered safe for swimming.
Enterococci
 3D rendered medically accurate illustration of an Enterococcus bacteria
3D rendered medically accurate illustration of an Enterococcus bacteria
Not unlike E. coli, Enterococci are bacteria that live in the intestinal tracts of warm-blooded animals, including humans, and therefore indicate possible contamination of streams and rivers by fecal waste.
Sources of fecal indicator bacteria such as Enterococci include wastewater treatment plant effluent, leaking septic systems, stormwater runoff, sewage discharged or dumped from recreational boats, domestic animal and wildlife waste, improper land application of manure or sewage, and runoff from manure storage areas, pastures, rangelands, and feedlots. There are also natural, non-fecal sources of fecal indicator bacteria, including plants, sand, soil, and sediments, that contribute to a certain background level in ambient waters and vary based on local environmental and meteorological conditions.
Enterococci are measured in MPN “Most Probable Number“, and are calculated from viable bacteria growing in a liquid medium; as opposed to E. coli which is measured in CFUs, or “Colony Forming Units”.
Key Takeaways:
In waterways, Enterococcus and E. coli are indicators of fecal pollution, either human or animal-derived.
Alkalinity measures the buffering capacity of water, or in other words, the waters ability to neutralize the acids that is comes in contact with. Water has the ability to do this by the use of a base. Let’s briefly go back to Chemistry 101 for a quick description of what an acid is and what a base is. An acid is a compound substance that when mixed with water will break down on the molecular level resulting in single hydrogen ions (H+). A base is also a compound substance, but will result in hydroxide ions (OH-) when mixed with water. When water comes in contact with more acids than bases, there will be more hydrogen ions floating around compared to hydroxide ions resulting in a lower pH. On the other hand, when there are fewer acids, there are less hydrogen ions and more hydroxide ions resulting in a higher pH.
Water uses available bases, such as bicarbonates, carbonates, and hydroxides to combine with the free floating hydrogen ions (acids) that have entered the water. These acids can enter water from several sources including rainfall and wastewater. If water does not have a sufficient supply of base compounds, it will not be able to maintain a steady pH level while being exposed to an acidic substance. Once the buffering capacity of water is used up then you will begin to see drops in the pH value.
We have discussed how acid enters the water, but how does the base get in there? It’s all in the rocks. Geology has a significant impact on the alkalinity of water. For example, in areas where the geologic composition contains carbonate (base) rich materials — such as limestone — the water tends to be more alkaline. By comparison, areas that contain carbonate poor soils — such as granite bedrock — have low alkalinity levels.
The Saco River Corridor Commission is committed to protecting public health and safety and the quality of life for the state of Maine. The commission regulates land and water uses, protects and conserves the region’s unique and exceptional natural resources, and prevents the detrimental impacts of incompatible development. The commission was established in 1973.